Friday, March 9, 2012
Practice Final Exam 2010 Answers
A few mistakes on this answer key:
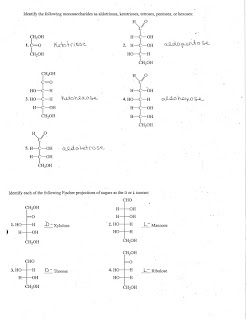
1. The haworth structure for fructofuranose should have CH2OH instead of H on the first carbon immediately right of the oxygen at the top of the ring.
2. On page 4 number 3a, the extended structural formula should include two lone pairs of electrons on each oxygen.
3. On page 4 number 3c, in the third step of the mechanism the CH3-CH2-O molecule should not directly remove the H from from the carboxyllic acid. You should first add a hydrogen to the CH3-CH2-O to make ethanol. Do this by drawing an arrow from the negatively charged oxygen to a hydrogen on the water molecule. This step will produce ethanol and -OH. Then use the -OH molecule to remove the H from the carboxyllic acid. Do this by drawing an arrow from -OH to the H on the carboxyllic acid.
4. On page 6 number 5b iii, the answer should be 3-methyl-6-nonanol.
(Disclaimer: I didn't make this answer key...)
Practice Final Exam 2011 Answers
Here's the answer key to the 2011 final exam. One thing to note: One page 4 where the resonance structures for CO3 are drawn out, you should draw arrows showing where the electrons move to make the next resonance structure. (i.e. draw an arrow from the electrons on one of the singly bonded oxygens showing the formation of a double bond, and draw a second arrow from the double bond forming a lone pair on the oxygen)
Quiz Section Exercise: Esterification
Oops! That last problem on this worksheet isn't finished! If you came to quiz section, then we did it correctly in quiz section. Notice, the hydrogen is missing from the wrong oxygen. You have to do two more steps to finish this problem. The first step is where negative oxygen takes a hydrogen from water. The second step is where the hydroxide ion takes off the hydrogen from the carboxyllic acid. Sorry!
Friday, March 2, 2012
Practice Final Exam
In case you want to start early to study for the final exam, here is a practice test. This is the final exam from 2010. You can also try the final exam from 2011 that is posted on the main chem 220 website.
Friday, February 17, 2012
List of Reactions
Here's a list of the reactions you have learned so far. Make sure you can write the products and know the catalysts that are necessary for each reaction. The starred reactions are the ones that you can possibly write a mechanism for.
addition reactions: adding a molecule to an alkene to get an alkane product
- hydrogenation *
- halogenation *
- hydrohalogenation (Markovnikov's rule) *
- hydration (Markovnikov's rule) *
polymerization *
reactions of aromatic compounds: adding a functional group to a benzene ring
- halogenation
- nitration
- sulfonation
reactions of alcohols: reacting with the -OH group
- dehydration (Saytzeff's rule; the only way you know how to make an alkene) *
- formation of ethers *
- alcohol oxidation (consider for primary and secondary alcohols)
reactions with thiols: reacting with the -SH group
- oxidation (creating disulfide bonds) *
aldehydes and ketones: reacting with C=O group
- oxidation
- tollens' test
- benedict's test
- reduction
- addition
- of water *
- hemiacetal formation (including cyclic acetals) *
- acetal formation *
Thursday, February 16, 2012
Extra Practice Exam
We'll be going over Practice Exam 2 from 2011 in quiz section tomorrow, which you can find under "exams" on the main Chem 220 website. However, here's another practice exam (from 2010) for your studying pleasure.
Subscribe to:
Comments (Atom)